
By AGC Biologics
For drug sponsors, setting up your CGT product for regulatory viability and commercial success means investing the time and budget to empower process design, as well as on-time planning of process performance qualification (PPQ) and continued process verification (CPV) activities.
Easier said than done. Consider that many sponsors are new to, or relatively inexperienced in, the CGT market. Often, they want to complete early development steps as quickly and with as little budget expenditure as possible. While shortcuts are difficult to create due to GMP requirements, a CDMO that offers sponsors a platform process and/or platform analytical methods provides sponsors significant advantages versus starting from scratch. These advantages can ease and accelerate process design, PPQ, and CPV.
Empower Process Design
Product and process development usually follow a formula: complete minimal activities for process suitability and safety, then move to GMP production for early clinical trials, the first major milestone, and use that to raise funds. Although this paradigm is unlikely to change, it's important to add activities early to enhance process knowledge.
Otherwise, when design and development shift to PPQ and regulators request data to populate the common technical document (CTD), that information often remains unavailable. In such situations, the sponsor may push the CDMO to generate additional data for the CTD while also forging ahead on PPQ — a complex, risky, and expensive endeavor. By completing a more comprehensive Stage 1, using three or four years to refine process design and process knowledge, a sponsor arrives at Stage 2 with a better understanding of the process and more robust documentation to justify PPQ activities, and they are in a safer position to start populating the CTD.
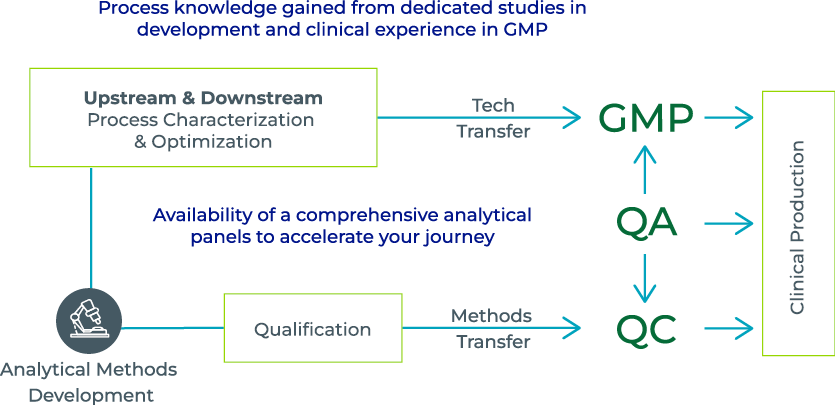
Parallelism between process and method development facilitates a path to successful clinical manufacture.
Generally, this task comprises defining and planning process characterization studies. Working with a CDMO that utilizes a platform process means many such studies are immediately at the client’s disposal, as is knowledge gained from studies conducted on similar processes. This is supplemented with more client- and product-specific characterization studies. From those studies and other development data, potential critical process parameters (CPPs) as well as their impact on critical quality attributes (CQAs) are determined.
Some studies can be accomplished through a qualified scaled-down model, representative of the full-scale process; other studies require higher-volume manufacturing. During process design, the CDMO may work with the sponsor to decide which studies are performed at which scale (as well as whether certain studies can be combined) and/or which studies depend upon completion of some prerequisite activity.
Wrapping up Stage 1 means designing a suitable process, gaining basic knowledge, and identifying a control strategy that is based on process understanding and ensures product quality (per ICH Q11: controls on material attributes, controls implicit in the design of the manufacturing process, in-process controls, controls on the drug substance).i Again, working with an organization that has established a platform process is invaluable. For example, the LVV processes at AGC have a control strategy already in place. While moving from process development to GMP manufacturing still requires some optimization, that effort is mainly to test the suitability of AGC’s process on the specific client product and to develop product-specific analytical methods.
Strategize Process Performance Qualification (PPQ) From the Start
Preparing a robust PPQ strategy entails solidifying process parameters and insulating your control strategy against commercial-level scrutiny. Additionally, advancing through PPQ requires that analytical methods progress from scientifically sound to fully validated. It is productive to view this strategy from a process perspective, an analytical perspective, and a methodological perspective.
The goal is to arrive at PPQ with the process almost locked and requiring few, if any, changes. Ideally, the process arrives at PPQ with several GMP batches completed for clinical proposals, and those have generated both positive product results and a certain level of process reproducibility.
More commonly, sponsors accept the risk of moving forward to PPQ with a limited number of GMP batches available, trying to stay ahead of demanding project timelines. A CDMO using a platform process offers advantages in this respect, since it can provide master batch record (MBR) to GMP production that is based on previous instances of the completed process.
In short, the sponsor may not be able to provide regulators with comprehensive documentation, but they can generate enough to kick off the PPQ campaign.
From an analytical and methodological viewpoint, an analytical platform can provide sponsors with ready-to-use, platform-validated methods that can be used to characterize the process, to verify impurities removed during purification, and to establish in-process controls (IPC), etc., for different projects.
CDMOs also support sponsors by developing or internalizing product-specific assays, allowing most analytics to be done in-house. This optimizes QC samples and speeds up release timelines. It is vital to begin developing potency methods in the early clinical phases, even if not mandated by regulatory bodies, because their establishment is a time-consuming process. A delay requires sponsors to commit additional time and cost during the PPQ stage for product-specific analysis and validation, along with post-PPQ responsibilities like data analysis and issue resolution, thereby straining their project timelines and budget.

How analytical methods follow process validation.
What Gaps Do You Need to Fill?
Designing and validating a CGT production process that is both scalable and repeatable at cGMP quality is complex, but your journey can be eased by following the right strategy, embracing platform processes to hit the ground running, and working with an experienced partner.
First, invest to empower Stage 1 activities; as the saying goes, pay now, or pay later. Second, start planning for Stage 2 PPQ activities at the outset of the project, so fewer unexpected obstacles pop up during PPQ. Third, constantly evaluate and amend your CPV strategy to account for study results, process changes, and/or updated analytics throughout the project. Finally, prioritize finding the right CDMO partner with a proven track record of success and demonstrable scientific expertise. A partner that offers in-house analytics and has the flexibility to quickly adapt as challenges arise during preparation for late-phase work and PPQ stages will help you save valuable time, money and resources.
To learn more, contact the authors and explore AGC Biologics’ cell therapy services, as well as its ProntoLVV™ and BravoAAV™ vector platforms.
RESOURCES
AGC Biologics to Manufacture First-ever FDA Approved Gene Therapy for Early-onset MLD, Orchard Therapeutics’ Lenmeldy™
Opportunities To Accelerate Allogeneic Cell Therapy (bioprocessonline.com)
Delivering LVV Material In An Accelerated Timeline (bioprocessonline.com)
New Scale Down Models To Get Vectors To GMP Stages (bioprocessonline.com)