1. Introduction
Cryopreservation is a critical component in the storage of cell-based therapies, ensuring product stability, cell viability, and product efficacy. As the cell and gene therapy field advances, understanding best practices, technological challenges, and trends in the industry is essential for maintaining manufacturing standards and regulatory compliance.
The latest survey developed and analyzed by the ISCT Cold Chain Management and Logistics Working Group, part of the wider Process Development and Manufacturing Committee, provides key insights into current cryopreservation practices. This includes the prevalence of controlled-rate freezers (CRF), challenges in system qualification, and the role of freeze curves in process monitoring. Additional topics are explored such as thawing procedures, scale up and out strategies, and how industry professionals are adapting to new technologies that are being developed to enhance process efficiency.
This blog summarizes the findings of the cryopreservation survey, offering a comprehensive look at cryopreservation practices in the cell and gene therapy industry.
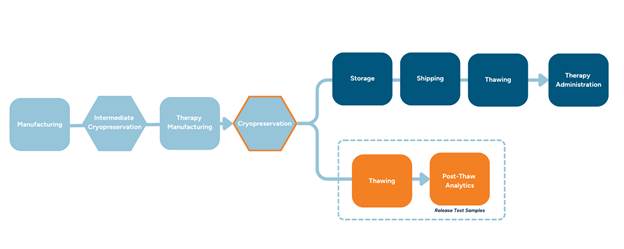
Figure 1: Cryopreservation Supply Chain Process Flow
2. Key Takeaway 1 - There is little consensus on how to qualify controlled-rate freezers and if different form factors should freeze together.
The majority of respondents indicated that they use controlled-rate freezing during the cryopreservation process. In the context of cGMP manufacturing, this allows a much broader set of documentation which can be incorporated into manufacturing controls and process monitoring. However, currently there is little consensus on how qualification of controlled-rate freezers should be carried out and the use of freeze profiles to inform better understanding of the process.
One observation of note from the survey, was that nearly 30% of respondents rely on vendors for system qualification. When this is a specific qualification based on intended use cases and profiles, this could provide a level of expertise that some users do not have on site. However, users should know that a system qualification by a vendor for unit specific performance (for example Factory Acceptance Testing) or validation during system development is often not representative of the final use case and requires an understanding of the vendor’s qualification profile(s)and how that relates to their boundary conditions. In the end, a qualification that is based on a profile only is likely to leave gaps in a user’s understanding of the impact on different kinds of samples and container types. A qualification should include a range of mass, container configurations, and temperature profiles. It is typically advisable to understand if a basic intended configuration works as needed but also, what other configurations may define the limit of performance.
Examples of profiles to evaluate can include:
● Full versus empty temperature mapping
● Temperature mapping across a grid of locations
● Freeze curve mapping across locations of different container types
● Mixed load freeze curve mapping
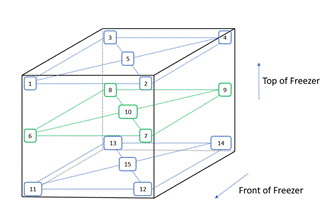
A typical temperature map might look like the following shown in Figure 2.
Figure 2: Typical Temperature Mapping Strategy
A second key observation is the limited use of freeze curves as part of the release process. A large number of respondents indicated that the freeze curves are not used for release, and instead rely on post-thaw analytics alone. As the CGT industry develops a deeper understanding of the important critical quality attributes (CQAs) of cell-based products and how to measure them, reliance on process-related data as an element of release becomes less important. However, even in the case that CQAs are well-established, process data monitoring should be considered as an important element of manufacturing controls. Freeze curves can provide information about the ongoing performance of the CRF system itself and identify why a sample did not perform as expected in post-thaw analytics. Establishing action or alert limits for curves can be used to identify changes in CRF performance, potentially alerting users of the need to intervene before a critical failure.
For reference, CRF users may find helpful information in the ISPER guideline:
Good Practice Guide: Controlled Temperature Chambers 2nd Edition, 2021. (https://ispe.org/publications/guidance-documents/ispe-good-practice-guide-controlled-temperature-chambers-2nd-edition#:~:text=The%20controlled%20temperature%20chambers%20discussed,and%20criticality%20of%20product%20stored).
3 Key Takeaway 2 – There is a consensus that cryopreservation (freezing process and CryoMedia composition) and post-thaw analytics face the most challenges and have the most resources dedicated to it.
Often underestimated, the thawing process plays an important role in maintaining the critical quality attributes of cell-based advanced therapies.
Non-controlled thawing can cause osmotic stress, intracellular ice crystal formation and prolonged exposure to DMSO, leading to poor cell viability and recovery. This is true for cryopreserved starting material, QC sample thawing in the context of manufacturing, or the Drug Product thawing prior to administration to the patient.
The thawing of cell products at the bedside requires well-trained staff, however it is frequently poorly regulated. Conventional water baths are not GMP-compliant and represent a source of contamination risk, rely on manual operation, and are subject to routine cleaning and validation efforts in the clinical setting. This has led to both production and clinical stakeholders to introduce controlled thawing devices into their routine.
A key factor in successful cryopreservation process is definition of the optimal temperature profile for both freezing and thawing. The established good practice for thawing includes the warming rate of 45°C/min. Recent publications supported by evidence point out relevance of different (slower or higher) warming rates for T cells, under conditions that the cooling rates are “slow” : −1 °C/min or slower. Nevertheless, control over the warming rates and robustness of the thawing procedure remains crucial for reproducible GMP and bedside thawing.
4 Key Takeaways 3 and 4 – Of those who completed the survey, 60% are using default CRF, but there are a wide variety of challenging cell types that need optimized conditions such as CAR-T cells, engineered cells, iPSC differentiated cells. Additionally, adoption rate of controlled-rate freezing for cryopreservation is high, as opposed to passive freezing.
87% of survey participants reported using controlled-rate freezing for the cryopreservation of cell-based products in their current practice. Of those that use passive freezing (the remaining 13%) 86% have products exclusively in the earlier stages of clinical development (up to phase II). This information suggests a high prevalence of controlled-rate freezing, especially for late stage and commercial products, although it may be related to the selection bias surrounding a voluntary cryopreservation survey.
How is controlled rate freezing better than passive freezing? Controlled-rate freezers (CRFs) allow us to control the rate of cooling within a product’s tolerance and provide automated solutions for documentation. The rate of cooling before nucleation (related to chilling injury and cryoprotective agent (CPA) toxicity), the temperature of ice nucleation (related to osmotic stress and intracellular ice formation), the rate of cooling after nucleation (related to dehydration and intracellular ice) and before colloidal glass transition of the cell-containing liquid fraction, and the final sample temperature at the end of freezing run before transfer into downstream unit operation (e.g., cryogenic freezer for storage) are all process parameters that can be defined by the user and controlled by the freezer. When relevant and feasible, controlled rate freezing can be an effective tool in controlling the quality and consistency of the cryopreserved product.
Table 1: Advantages and disadvantages of CRF or passive freezing methods
|
|
Controlled Rate Freezing
|
Passive Freezing
|
|
Advantages
|
● Control over critical process parameters (e.g., cooling rate) and their impacted critical quality attributes (e.g., cytokine release)
|
● Simple, one-step operation
● Low-cost, low-consumable infrastructure
● Low technical barrier to adoption
● Ease of scaling
|
|
Limitations
|
● High-cost, high-consumable infrastructure
● Specialized expertise required for use and optimization
● Bottleneck for batch scale-up
|
● Lack of control over critical process parameters and their impacted critical quality attributes
● Advanced pre-freeze or thawing technology potentially required to mitigate freezing damage
|
Is the controlled rate freezer’s default profile good enough? CRFs typically come with default (standard) profiles that may work for a wide variety of products. In other cases, an optimized profile developed independently or modified based on a default profile may be necessary for the effective cryopreservation of certain specialized or sensitive cells and for the utilization of certain CRF load configurations or specialized primary containers. One or a group of CRF process or post-thaw analytics metrics may be used to qualify a default CRF profile or drive the CRF profile optimization. In our survey, 60% are using default profiles, fairly representing the entire spectrum of clinical stages and industry sectors. 33% have dedicated the most resources and R&D efforts towards freezing process development. Those using an optimized CRF profile or experiencing challenges with a default profile are typically working with iPSCs, hepatocytes, cardiomyocytes, photoreceptor and other solid tissue cell types, macrophages, B cells among a few specific cases of T-cells, NK-cells, HSCs and MSCs. Whether each CRF parameter is critical to the quality of the cryopreserved product should be determined on a case-by-case basis for each product and may change depending on cell type, cell harvest condition, CPA formulation, primary container, and critical quality attribute of interest.
Should we always use controlled rate freezing? As going from passive to controlled-rate freezing changes many potentially critical process parameters, adopting controlled-rate freezing early on in clinical development can avoid the challenging effort of making a significant manufacturing change and establishing comparability subsequently. However, controlled-rate freezing is resource-intensive, in terms of infrastructure (CRF instrument), operating costs (liquid nitrogen consumption, staffing), and process development (experimentation, expertise), and may present a bottleneck to batch scheduling and process efficiency in the overall manufacturing workflow. Advanced, fit-for-purpose cryopreservation technologies in terms of pre-conditioning and CPA formulation may present novel opportunities that enable an adequate use of passive freezing without compromising the quality or consistency of cryopreserved cells. While process control tips the scales towards controlled-rate freezing, process efficiency and batch scale-up may move the needle of cryopreservation in specific cases towards passive freezing.
5 Key Takeaway 5 - The industry views scaling as a major hurdle.
Scaling the cryopreservation was identified as a major hurdle for the industry to further support the advancement of cell and gene therapies, with the majority of respondents (22%) identifying “Ability to process at a large scale” as the biggest hurdle to overcome for cryopreservation. As more therapies move closer to commercialization, scaling techniques and technologies will be required to ensure that therapies are manufactured and cryopreserved in a manner that is efficient while maintaining the critical quality attributes of the therapy.
Figure 3: Survey Response on biggest hurdle to overcome for cryopreservation in CGT
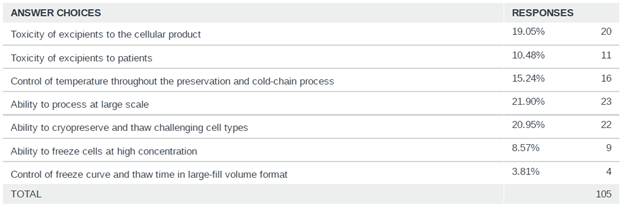
Additionally, the majority of respondents (75%) cryopreserve all units from an entire manufacturing batch together, indicating that manufacturing scale is commonly and sufficiently small in the industry, where dividing a manufacturing batch into sub-batches for cryopreservation is a less common (25%) need and practice. For a given batch size off the manufacturing line, cryopreserving the entire batch together has greater variance in time between start and end of each batch, whereas cryopreserving sub-batches staggered or sequential in start time and potentially using different units of freezers has greater risk in freezing process reproducibility between the sub-batches.
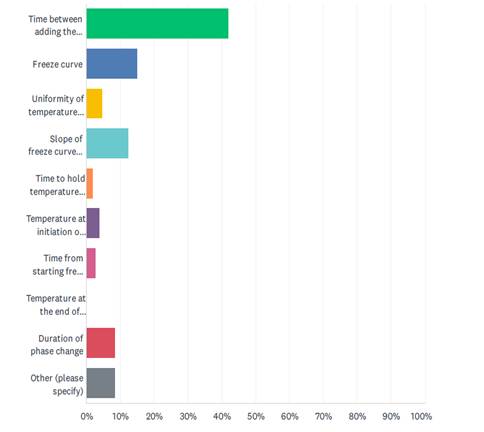
Scaling, in regards to cryopreservation, presents several challenges, including how cytotoxicity is monitored and managed, batch size considerations, time between batch sizes (sub-batching), and the time between addition of the cryoprotective agent and freezing. The survey also showed that the majority (42%) regard the time between adding the cryoprotectant and starting the freeze run as the most critical parameter during cryopreservation. As optimal CPA incubation time is a balance between uniformity of CPA penetration and cytotoxicity of the CPA, effectively and consistently controlling that time will become more difficult as manufacturing scales up. Specifically, the survey indicates that the industry faces scaling challenges on the scale up to 1500 vials, 500 bags, 60 liters, and 2 shifts.
Figure 4: Survey Response on the most Critical Parameter during Cryopreservation
CPA formulation is an important factor determining the window of opportunity when scaling. Besides potential cytotoxicity of DMSO, the composition of cryopreservation solution can impact cell health in terms of osmotic stress, oxidative stress, membrane fluidity, nutrient deprivation, etc. Strategies such as reducing DMSO concentration, avoiding cell-intolerant compounds, balancing osmosis with multi-component formulation, and reducing the formulation temperature can extend the time window that a cell-based product may tolerate between the point of formulation, through fill-finish, to the start of freezing. Moreover, the time window is determined by the critical quality attribute of interest for the cell-based product of interest, where the longer time lag between start and end of cryopreservation batch and the greater risk of process variability between sub-batches in scale-up may or may not affect the quality metric at all, and the post-thaw analytics method may or may not be sensitive enough to capture any changes in the critical quality attribute caused by the scale-up. In the next installment of our survey blog series, we will dive into the findings from the 2024 Post-Thaw Analytics survey.
Through ISCT, the Cold Chain and Logistics Management Working Group intends to explore these key considerations and share with the industry techniques, best practices, and effective strategies from leading industry experts through webinars, roundtable discussions, and blog posts.
References:
Cottle, C., Porter, A. P., Lipat, A., Turner-Lyles, C., Nguyen, J., Moll, G., & Chinnadurai, R. (2022). Impact of cryopreservation and Freeze-Thawing on therapeutic properties of mesenchymal Stromal/Stem cells and other common cellular therapeutics. Current Stem Cell Reports, 8(2), 72–92.
Gibb, S. L., Matthay, M., Nizzi, F., Marlowe, M., Jones, B., & Pati, S. (2015). Exposure of mesenchymal stem cells to DMSO post-thaw affects cell viability and signaling. Cytotherapy, 17(6), S46.
Baboo, J., Kilbride, P., Delahaye, M., Milne, S., Fonseca, F., Blanco, M., Meneghel, J., Nancekievill, A., Gaddum, N., & Morris, G. J. (2019). The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Scientific Reports, 9(1).
#IndustryMemberNews