Dr. Emily Blyth, Associate Professor, Haematologist
Westmead Hospital, Westmead Institute for Medical Research, University of Sydney
Sydney, Australia
Dr Selmir Avdic, Production Manager
Westmead Institute for Medical Research, University of Sydney
Sydney, Australia
Disclaimer: EB declares advisory roles for Astellas, MSD, Abbvie, Novartis, BMS, Bastion Education and IQVIA. Research funding from MSD.
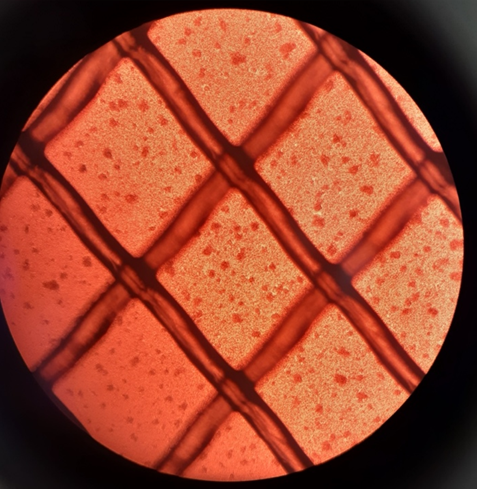
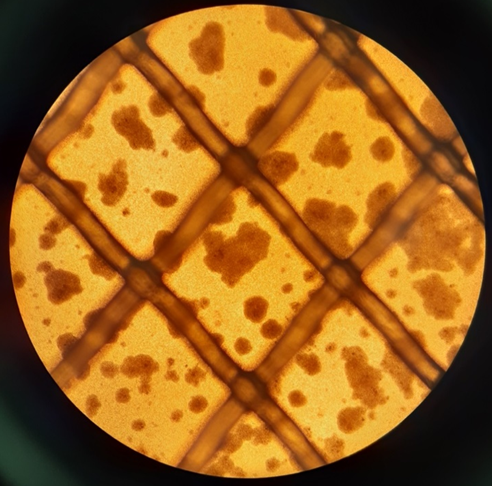
SARS-CoV2 virus specific T cells (VST) expanding in culture in a bioreactor on day 5 (media supplementation 1, left image) and day 12 (harvest, right image). Images taken on Olympus CKX41 microscope with Olympus DP21 camera; Image credit Kelly Wray.
What? Pathogen specific T cells (PSTs) are T cells whose receptor is specific for an epitope from an infectious organism. They can be stimulated by various antigen sources and purified, or ex vivo expanded, for a therapeutic PST product. This was first performed successfully and applied in human trials in the 1990s in patients with immune deficiency from transplantation who had virus-driven diseases (1-5).
In the laboratory, PSTs are isolated from starting material (usually peripheral blood mononuclear cells or the T cells in a leukocyte apheresis) by utilising the natural interactions between epitope and T cell receptor (TCR). T lymphocytes are activated by interaction with antigen presented on the major histocompatibility complex (MHC) molecules of antigen-presenting cells (APC). Exogenous proteins are taken up by the APC and processed into peptide fragments. These bind MHC class II molecules for interaction with T cell receptor (TCR) of CD4+ T lymphocytes leading to activation and clonal expansion. Endogenous antigens (including the products of viral reproduction in infected cells) are loaded onto MHC class I molecules for presentation to CD8+ T cells. Engagement of the TCR/CD8 complex results in CD8+ T cell activation, killing of infected cells and other functions such as production of inflammatory cytokines (1-3).
The first pathogens to be targeted by ex vivo expanded PSTs were Epstein-Barr virus (EBV) and cytomegalovirus (CMV) (4-8). At the current time, the approach has been generalised to many pathogens, including adenovirus, polyomaviruses (BK and JC), varicella virus, respiratory viruses (influenza, respiratory syncytial virus, parainfluenza), herpes simplex virus, hepatitis B and others (9-15). Fungus specific T cells are also under development as invasive fungal infections remain an important pathogen in immunosuppressed hosts (16, 17). SARS-CoV2 specific T cells appear to be important in the progression of COVID-19, and ex vivo isolated SARS-CoV2 specific T cells have been administered to several recipients and are under development in several centres, including our own (18-22).
Why? Cellular immune deficiency leads to the development of potentially life-threatening opportunistic infections, primarily with viral and fungal pathogens, and to virus-driven malignancies such as EBV post-transplant lymphoproliferative disease (PTLD). The reconstitution of cellular immunity via adoptive transfer of ex vivo isolated cellular immune effectors has been explored as a way to address this clinical problem directly. A number of phase I and II studies in humans have shown this to be feasible. Safety and efficacy data from these clinical studies are encouraging with no evidence of major adverse effects, including graft versus host disease (GvHD; 23). PSTs appear to reduce the progression of viral infection when used prophylactically and to be able to salvage treatment-resistant infections or virus-driven malignancy (7, 24-28). The goal of this approach in the transplant setting to facilitate immune reconstitution is a cellular therapy product that can restore immunity to pathogens that may cause illness or malignancy while minimising the risk to the recipient of alloreactivity associated with GvHD. The majority of clinical trials have been performed in a haemopoietic stem cell transplant (HSCT) setting where stem cell donor-derived products intended for the transplant recipient are straightforward to manufacture but are only available for a single recipient, and the approach is not generalizable to situations where there is no donor. To address this, cryopreserved cell banks have been generated from “third party” donors (not the transplant donor or recipient, but a third individual) where the T cell products can be prepared in advance and used in time of clinical need (25-27, 29). Third party cells can be manufactured in larger batches and have a significant advantage in terms of logistics and probably cost-effectiveness. In addition, they can be utilised in non-transplant settings (30) and as starting material for gene modified products with non-pathogen targets (31).
Who? The majority of clinical studies were performed in academic centres in the first decade of the development of therapeutic PSTs. More recently, further development has been undertaken by commercial entities towards commercial PSTs becoming available for general use.
Where? PSTs are under investigation worldwide. Academic centres pioneered the development of cell isolation methods and clinical translation. More recently, several biotechnology companies have developed PST products that are in commercial development. No PST has yet received authorisation for clinical use in any jurisdiction. Ebvallo™ (tablecleucel; Atara Biotherapeutics Inc.) has recently received a positive evaluation from the European Medicines Agency’s Committee on Human Medicinal Products for use in EBV PTLD.
How? PSTs have been manufactured for clinical use for more than 25 years but methods for isolation and/or ex vivo expansion have evolved over time. Initially, T cells were stimulated to activate and expand in culture using autologous antigen presenting cells (EBV transformed lymphoblastoid cell lines (LCLs) or dendritic cells), and in some cases enrichment was performed by limiting dilution cloning (5, 8, 32). Both of these processes took up to 3 months per individual cell product. As the field has developed, consumables such as bioreactors, clinical grade peptides, and antigen expressing viral vectors have increased the speed and robustness of ex vivo “stimulate and expand” methods. Cell selection methods using the expression of activation markers or by cell capture based on cytokine production and using MHC/eptiope constructs to directly bind known T cell receptors have allowed rapid isolation and direct infusion to patients, without the need for ex vivo expansion (9, 33, 34).
Did you know that… In vivo tracking of the fate of clones that have been infused to recipients has been a challenge over the history of adoptive T cell immunotherapy. An early trial used a marker gene to identify transferred clones derived from transplant donors that were administered to patients with EBV infection (35). These clones were shown to persist for up to 10 years by the identification of the marker gene by PCR. Gene marking solely for clone tracking is no longer acceptable to regulators so alternative methods such as tracking of unique T cell receptor variable sequences have been utilized (33, 36). Using this method, donor-derived clone persistence has been confirmed in several more recent studies. In contrast, third party derived PST products do not appear to engraft in most cases, despite evidence of clinical and immunological benefits.
References
- Delves PJ, Roitt IM. Roitt's essential immunology. Chichester: Wiley-Blackwell; 2011.
- Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nature Immunology. 2008;9(11):1244-52.
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nature immunology. 2003;4(9):835-42.
- Rooney CM, Smith C, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9-13.
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. The New England Journal of Medicine. 1995;333(16):1038-44.
- Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916-22.
- Blyth E, Clancy L, Simms R, Ma CK, Burgess J, Deo S, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745-58.
- Haque T, Wilkie GM, Taylor C, Amlot PL, Murad P, Iley A, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. The Lancet. 2002;360(9331):436-42.
- Feuchtinger T, Lang P, Hamprecht K, Schumm M, Greil J, Jahn G, et al. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp Hematol. 2004;32(3):282-9.
- Blyth E, Clancy L, Simms R, Gaundar S, OʼConnell P, Micklethwaite KP, et al. BK Virus-Specific T Cells for Use in Cellular Therapy Show Specificity to Multiple Antigens and Polyfunctional Cytokine Responses. Transplantation. 2011;92(10):1077-84.
- Gerdemann U, Keukens L, Keirnan JM, Katari UL, Nguyen CTQ, de Pagter AP, et al. Immunotherapeutic strategies to prevent and treat human herpesvirus 6 reactivation after allogeneic stem cell transplantation. Blood. 2013;121(1):207-18.
- Hafezi M, Bertoletti A, Tan AT. T cell immunotherapy in hepatitis B Virus related hepatocellular carcinoma. Hepatoma Research. 2018;4(5).
- Blyth E, Gaundar SS, Clancy L, Simms RM, Bilmon I, Micklethwaite KP, et al. Clinical-grade varicella zoster virus-specific T cells produced for adoptive immunotherapy in hemopoietic stem cell transplant recipients. Cytotherapy. 2012;14(6):724--32.
- Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, et al. Rapidly Generated Multivirus-specific Cytotoxic T Lymphocytes for the Prophylaxis and Treatment of Viral Infections. Molecular therapy. 2012;20(8):1622-32.
- Ma CKK, Clancy L, Deo S, Blyth E, Micklethwaite KP, Gottlieb DJ. Herpes simplex virus type 1 (HSV-1) specific T-cell generation from HLA-A1- and HLA-A2-positive donors for adoptive immunotherapy. Cytotherapy. 2017;19:107-18.
- Castellano-Gonzalez G, McGuire HM, Luciani F, Clancy LE, Li Z, Avdic S, et al. Rapidly expanded partially HLA DRB1-matched fungus-specific T cells mediate in vitro and in vivo antifungal activity. Blood Advances. 2020;4(14):3443 - 56.
- Deo SS, Gottlieb DJ. Adoptive T-cell therapy for fungal infections in haematology patients. Clin Transl Immunology. 2015;4(8):e40.
- Panikkar A, Lineburg KE, Raju J, Chew KY, Ambalathingal GR, Rehan S, et al. SARS-CoV-2-specific T cells generated for adoptive immunotherapy are capable of recognizing multiple SARS-CoV-2 variants. PLoS Pathog. 2022;18(2):e1010339.
- Kim N, Lee JM, Oh EJ, Jekarl DW, Lee DG, Im KI, et al. Off-the-Shelf Partial HLA Matching SARS-CoV-2 Antigen Specific T Cell Therapy: A New Possibility for COVID-19 Treatment. Front Immunol. 2021;12:751869.
- Martits-Chalangari K, Spak CW, Askar M, Killian A, Fisher TL, Atillasoy E, et al. ALVR109, an off-the-shelf partially HLA matched SARS-CoV-2-specific T cell therapy, to treat refractory severe COVID-19 pneumonia in a heart transplant patient: Case report. Am J Transplant. 2022;22(4):1261-5.
- Vasileiou S, Hill L, Kuvalekar M, Workineh AG, Watanabe A, Velazquez Y, et al. Allogeneic, Off-the-Shelf, SARS-CoV-2-specific T cells (ALVR109) for the treatment of COVID-19 in high risk patients. Haematologica. 2022.
- Leen AM, Myers GD, Bollard CM, Huls MH, Sili U, Gee AP, et al. T-cell immunotherapy for adenoviral infections of stem-cell transplant recipients. Annals of the New York Academy of Sciences. 2005;1062(1):104-15.
- Melenhorst JJ, Castillo P, Hanley PJ, Keller MD, Krance RA, Margolin J, et al. Graft Versus Leukemia Response Without Graft-versus-host Disease Elicited By Adoptively Transferred Multivirus-specific T-cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(1):179-83.
- Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis. 2008;40(1):63-7.
- Withers B, Blyth E, Clancy LE, Yong A, Fraser C, Burgess J, et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Advances. 2017;1(24):2193 - 205.
- Jiang W, Clancy LE, Avdic S, Sutrave G, Street JA, Simms R, et al. Third-party CMV- and EBV-specific T cells for first viral reactivation after allogeneic stem cell transplant. Blood Adv. 2022.
- Prockop S, Doubrovina ES, Suser S, Heller G, Barker J, Dahi P, et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplant. The Journal of clinical investigation. 2019.
- McLaughlin LP, Rouce R, Gottschalk S, Torrano V, Carrum G, Wu M-F, et al. EBV/LMP-specific T cells maintain remissions of T- and B-cell EBV lymphomas after allogeneic bone marrow transplantation. Blood. 2018;132(22):2351-61.
- O'Reilly RJ, Prockop S, Hasan A, Doubrovina ES. Therapeutic advantages provided by banked virus-specific T-cells of defined HLA-restriction. Bone Marrow Transplantation. 2019;54(Suppl 2):759 - 64.
- Pender MP, Csurhes PA, Smith C, Douglas NL, Neller MA, Matthews KK, et al. Epstein-Barr virus-specific T cell therapy for progressive multiple sclerosis. JCI insight. 2018;3(22):824.
- Cruz CRY, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965-73.
- Heslop HE, Ng C, Li C, Smith C, Loftin S, Krance R, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Medicine. 1996;2(5):551-5.
- Neuenhahn M, Albrecht J, Odendahl M, Schlott F, Dössinger G, Schiemann M, et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia. 2017;31(10):2161 - 71.
- Sutrave G, Blyth E, Gottlieb DJ. Cellular therapy for multiple pathogen infections after hematopoietic stem cell transplant. Cytotherapy. 2017;19(11):1284 - 301.
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925-35.
- Gottlieb DJ, Sutrave G, Jiang W, Avdic S, Street JA, Simms R, et al. Combining CD34+ stem cell selection with prophylactic pathogen and leukemia directed T-cell immunotherapy to simultaneously reduce graft versus host disease, infection, and leukemia recurrence after allogeneic stem cell transplant. Am J Hematol. 2022.
#ScientificSpotlight
#Scientific Spotlight
#ScientificSpotlight