
Sita Somara, PhD, MBA
RTI International
United States
Very random topics, right? But what I am trying to imply or bring out here is mainly the role that Artificial Intelligence can play in curbing the Healthcare cost of expensive treatments!!
Artificial Intelligence (AI) has revolutionized the way we interact with technology and has enormous potential to transform many areas of our lives. Among all the areas, AI has the potential to transform many aspects of healthcare, including the translation process of drug discovery and development.
Usually, for drugs to move from discovery to commercialization, it can take up to 15 or more years and costs around US$2.8 billion. The cost of the treatment soars as it covers the cost to support the research, development and manufacturing of the drug, which keeps rising based on the market. The cost of present treatment regimens, mainly the cell and gene therapies, is astronomically high. For example, Zolgensma, a gene therapy, costs US$2.1 million for a single dose. Though the costs are extremely high, the soothing features are that most of these treatments are one-time treatments, more effective and personalized, while the other conventional drugs developed are one-size fit all.
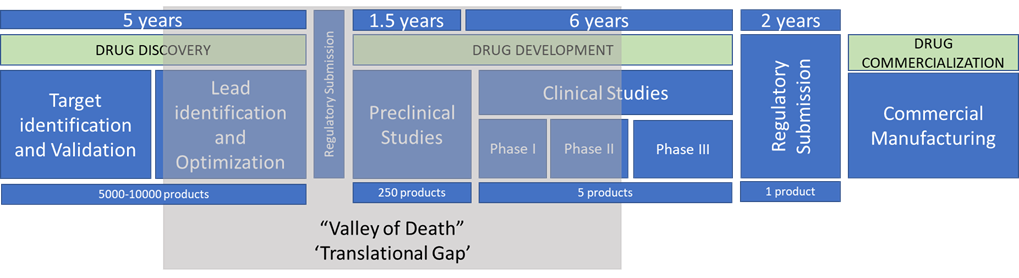
The prime focus of the translational process is to ensure that the discoveries which progress into clinical trials have the highest possible chance of success in terms of both safety and efficacy. The translational process is the most complex and riskiest industrial process owing to its high level of vulnerability to any slight changes in material, environment or process that can potentially alter the quality of the drug and nullify its efficacy. The three major phases of the process for discovery becoming a new therapy are (i) drug discovery consisting of target identification, target validation, lead identification and optimization, (ii) drug development that includes preclinical tests and clinical trials and (iii) drug manufacturing for commercialization that includes releasing the drug into market which would benefit the patient population at large.
Despite all the efforts made to optimize the translational process, it still faces challenges making it difficult to translate scientific discoveries into real-world treatments. Discoveries on the path of translation can get stuck or fail in the “valley of death” also generally known as the “translational gap” at any stage from discovery to development phase. The Valley of Death refers to the stages in translational process where promising scientific discoveries must be translated into actual drugs that can be tested in clinical trials and eventually brought to market. This is a difficult and expensive process, and many promising discoveries fail to make it through this stage. It is reported that of 5,000 - 10,000 compounds that enter the pipeline for drug development, 250 may reach preclinical development stage while only 5 would move to phase 1 trial with only one receiving market approval. Thus, only very few drugs or therapies make it all the way from discovery to clinical to commercial market with 80 to 90% of them failing owing to several different factors. Apart from the factors that fall under either technical, financial, or economic challenges, major cause of failure are lack of effectiveness and poor safety profiles from failed prediction from preclinical and animal studies. Surviving and crossing the Valley of Death will need deeper understanding of the drug effectiveness in the initial discovery phase and lot of clinical data mining along with strong collaborations among different stakeholders, including innovators, researchers, funders, sponsors, and practitioners.
Traditional drug development relies profoundly on human-derived rational and effort to identify the functional mechanisms of diseases, identify druggable targets, and design lead compounds to hit the targets with many potential drug candidates failing to make it to market due to efficacy or safety concerns. Despite progress in understanding human diseases and the advances in biotechnology, the search for novel therapeutics remains a time-consuming and costly process. Early elimination of the ones that are likely to fail can substantially decrease the overall cost of developing new products. While the healthcare industry is rapidly adopting digital developments, the one reported with the most potential is AI. With the recent tremendous success of AI in various domains, AI-based drug development is poised to become a revolutionary force in the pharmaceutical sector and is expected to fundamentally change the traditional trial-and-error design process.
AI can be used at different stages of the drug development process, from discovery to development to commercialization in different capabilities. With its ability to analyze large amounts of data and identify patterns, AI has the potential to accelerate the drug discovery process by enabling scientists to identify promising drug candidates more efficiently and accurately. AI-powered drug discovery tools use machine learning algorithms to analyze and learn from vast amounts of data, including scientific literature, clinical trial data, and genomic data. At discovery phase, AI is thought to curb costs by as much as 70%. These tools can quickly identify potential drug candidates and predict their efficacy, toxicity, and side effects. During the preclinical phase that involves testing on animals, utilizing AI seems to enable researchers to more quickly and successfully predict how a drug might interact with the animal model. AI-based tools can help optimize clinical trial design, identify patients most likely to benefit from a particular treatment and monitor patient outcomes in real-time. AI can help clinical trials run smoothly and facilitate participant monitoring while generating a larger set of data in parallel as the trial is ongoing with more accuracy and aid in participant retention by personalizing the trial experience. This can help reduce the time and cost of clinical trials and improve the chances of success for new drugs. By leveraging AI-powered drug discovery tools, researchers can identify promising drug candidates more quickly and efficiently, optimize production processes, personalize the treatment, and develop much more effective treatment, reducing the time and cost required to bring new drugs to market. The cost of developing new drugs is substantial, and these costs are often passed on to patients in the form of higher drug prices. By leveraging AI power, translation process can speed up, healthcare costs can be reduced, and drugs can be made more affordable for patients, and patient outcomes can be improved.
One of the main challenges in using AI for drug discovery is the need for high-quality data. AI algorithms rely on large datasets to identify patterns and make predictions. In the case of drug discovery, this means that there must be a large amount of high-quality data available on the biological mechanisms underlying disease, as well as data on the properties of potential drug candidates. This can be a significant barrier, as much of the data needed for drug discovery is still proprietary and not widely available. Another challenge is the need for regulatory approval. The drug development process is highly regulated, and any new drug must undergo rigorous testing to ensure its safety and efficacy. This process can be lengthy and expensive, and it can be difficult to convince regulators to approve a drug based on AI-based discoveries alone.
Despite these challenges, the potential benefits of using AI in drug discovery are significant. By accelerating the drug discovery process, AI has the potential to bring new treatments to patients more quickly and at a lower cost. However, it will take continued investment and collaboration between researchers, industry, and regulators to overcome the challenges and realize the full potential of AI in drug discovery and development.
What are your thoughts on AI-powered Translation Process?
References
Al Natsheh, Anas & Gbadegeshin, Saheed Adebayo & Ghafel, Kawtar & Mohammed, Omar & Koskela, Ashten & Rimpiläinen, Antti & Tikkanen, Joonas & Kuoppala, Antti. (2021). THE CAUSES OF VALLEY OF DEATH: A LITERATURE REVIEW. 10.21125/inted.2021.1943.
Calza, F., Ferretti, M., Panetti, E. and Parmentola, A. (2021), "Moving drug discoveries beyond the valley of death: the role of innovation ecosystems", European Journal of Innovation Management, Vol. 24 No. 4, pp. 1184-1209. https://doi.org/10.1108/EJIM-11-2019-0342
Chen W, Liu X, Zhang S, Chen S. Artificial intelligence for drug discovery: Resources, methods, and applications. Mol Ther Nucleic Acids. 2023 Feb 18;31:691-702. doi: 10.1016/j.omtn.2023.02.019. PMID: 36923950; PMCID: PMC10009646.
Gamo NJ, Birknow MR, Sullivan D, Kondo MA, Horiuchi Y, Sakurai T, Slusher BS, Sawa A. Valley of death: A proposal to build a "translational bridge" for the next generation. Neurosci Res. 2017 Feb;115:1-4. doi: 10.1016/j.neures.2016.11.003. Epub 2016 Nov 19. PMID: 27876581; PMCID: PMC5477974.
Seyhan, A.A. Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles. transl med commun 4, 18 (2019). https://doi.org/10.1186/s41231-019-0050-7